Multiple sclerosis (MS) is a leading cause of non-traumatic disability among young adults in North America and Europe and affects over 2.5 million people worldwide. Fortunately, for relapsing forms of MS there are now over a dozen treatment options, which we know can be effective at stopping relapses and slowing the accumulation of disability. What we don’t know is which treatment is best for different people.

Overview
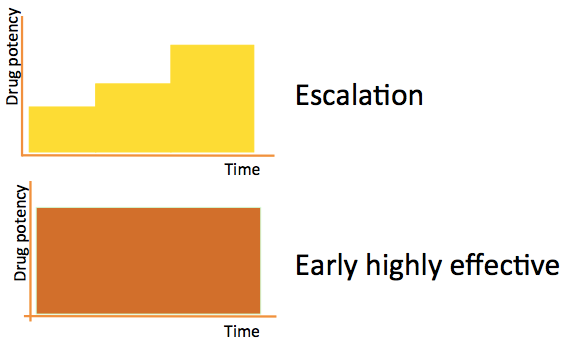
DELIVER-MS is a study that will be performed in 24 centers across the UK and US. The study will show whether starting treatment with a highly effective disease modifying therapy (DMT) improves the prognosis for people with MS. Currently it is not known if this is any better or worse than an escalating approach, where people are only taking stronger treatments if they are necessary to control their symptoms.
Population
The DELIVER-MS study will recruit people with relapsing-remitting MS who have not previously received any DMT. The study is designed to reflect clinical practice in MS clinics. There is an age limit of 55 years given that most people are diagnosed with relapsing MS at a younger age. The study will enroll people who experienced their first symptoms within the last 5 years to ensure they will get the most benefit from immunomodulating DMTs, mirroring regular clinical practice. We will enroll a total of 400 people with MS into a randomized study, and a further 400 into an observational study.
Study Design
Patients will be followed in the DELIVER-MS study for a total of 3 years. Those in the randomized part of the study will be told which initial DMT approach their neurologist should take, but their neurologist and the patients together will be free to decide which medication within that approach they choose. Once a person with MS is enrolled in the study and commences their first DMT, any subsequent changes that are required to the DMT will be made at the discretion of their treating neurologist, in just the same way as in routine clinical settings.
You can read more about the study protocol here.
Aims
This study will:
- Determine whether starting on a highly effective treatment is more effective than an escalation approach in slowing brain volume loss over 36 months
- It will also assess which approach is more effective at improving patient reported outcomes (PRO) and clinical measures, and the safety and tolerability of each approach.
How will this help people with MS?
At present, people with MS and their neurologists do not know which is the best treatment approach to take early in MS. Data from DELIVER-MS study will help weigh up the potential benefits and risks of starting treatment with highly effective DMTs. The data will help develop guidance for patients and neurologists on the use of existing and new treatments.