Study Summary

Proposal Title:
Determining the Effectiveness of earLy Intensive Versus Escalation approaches for the treatment of Relapsing-Remitting Multiple Sclerosis (DELIVER-MS).
Short Title:
DELIVER-MS
Principal Investigators:
Daniel Ontaneda (US PI)
Nikos Evangelou (UK PI)
Project Manager:
US: Sarah Planchon Pope
UK: Harriet Howard
Study Statisticians:
Douglas Gunzler
Stephen Gerry
MRI Analysis:
Kunio Nakamura
Medical Monitor:
Mary Alissa Willis
Study Management:
Cleveland Clinic
Data Coordinating Center:
Cleveland Clinic
MRI Reading Center:
Cleveland Clinic Foundation MS MRI Analysis Center
Funding:
PCORI
Patient care covered by insurance (US) or NHS (UK)
Non patient care/ analysis, testing investigator effort, database management, patient engagement sessions, analysis, to be covered by award
IND Number:
Waived
IND Holder:
Not Applicable
ClinicalTrials.gov ID
NCT 03535298
Study Phase:
Phase IV

Study Design:
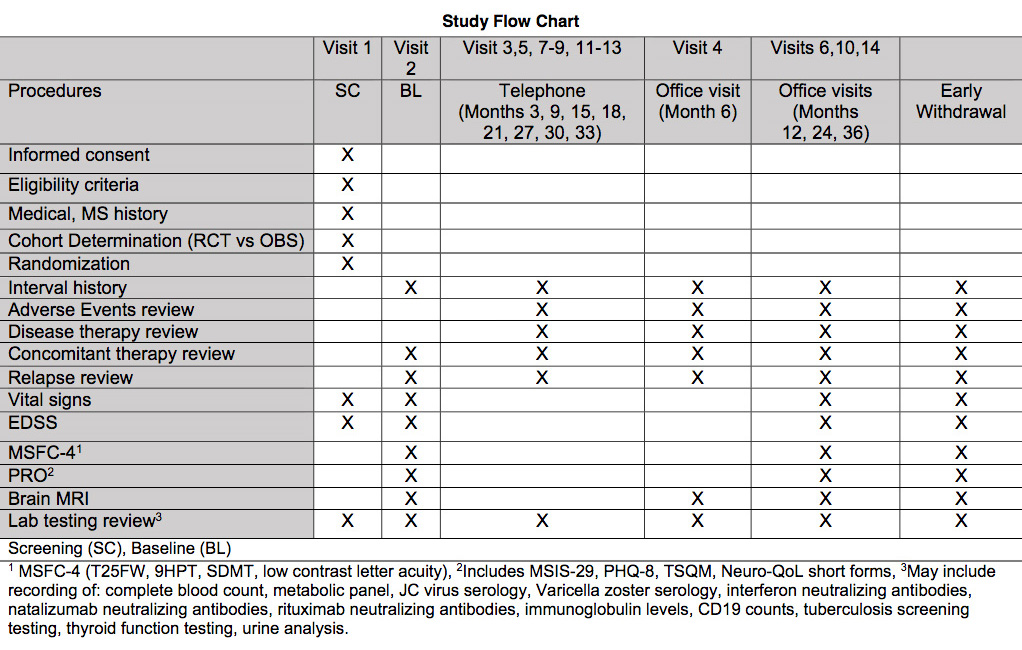
Multi-center pragmatic comparative effectiveness randomized clinical trial with additional-parallel observational cohort.
The DELIVER-MS study seeks to answer the important question: Does early treatment with highly effective DMT improve the prognosis for people with MS? This is an area of significant controversy and no data currently exist to guide treatment choices for patients and clinicians. The study results will help guide overall treatment philosophy and will be applicable not only to a wide range of existing therapies but also to new therapies, meeting a significant unmet need in patient decision making and aiding the decision for medication approval by third parties.
Objectives:
Primary Objective
To determine whether an EHT approach to DMT, defined as use of one of four monoclonal antibodies (alemtuzumab, natalizumab, rituximab, or ocrelizumab) as first-line therapy, is more effective than an escalation of treatment approach in reducing normalized whole brain volume loss measured using MRI in PwRRMS from Baseline to Month 36.
Secondary Objective
To determine the efficacy of an EHT approach as compared to an escalation approach as reflected by the following:
- Brain volume loss using MRI from month 6 to month 36.
- Proportion of participants with a multidimensional composite comprised of EDSS progression (>5 points for those with EDSS of 0 at Baseline, ≥1.0 for those with EDSS of 0.5-5.0 at Baseline, and >0.5 points for those with EDSS above 5.0 at Baseline), 20% change in MSFC-4 subcomponents (T25FW, 9HPT), 10% in SDMT or, 1 line change in LCLA confirmed over 12 months.
- Baseline to 36 month change in MSIS-29
- Baseline to 36 month change in Neuro-QoL
To determine the safety of an EHT approach as compared to an escalation approach as reflected in the following:
- Proportion of participants with SAEs
- Rate of SAEs
- Proportion of participants with discontinuation
- Cumulative on therapy TSQM Response scores
Exploratory Objectives
To determine the efficacy of an EHT approach as compared to an escalation approach as reflected by the following:
- Baseline to 12 month brain volume loss on MRI
- Month 12 to 24 brain volume loss on MRI
- Month 12 to 36 brain volume loss on MRI
- Month 24 to 36 brain volume loss on MRI
- T2 lesion volume change, Baseline to 36 month from brain MRI
- T2 lesions volume change, 6 month to 36 month from brain MRI
- T1 lesion volume change, Baseline to 36 month from brain MRI
- T1 lesion volume change, 6 month to 36 month from brain MRI
- Cumulative number of GdE lesions over 36 months
- GMF change, Baseline to 36 months from brain MRI
- GMF change 6 months to 36 months from brain MRI
- Annualized relapse rate (ARR)
- Time to first relapse
- Proportion of patients with NEDA (absence of N/E T2, GdE lesions, and EDSS [progression/as described for secondary endpoint] over 36 months)
- Comparison of all efficacy/safety measures in RCT and observational cohort
Number of Participants:
400 PwRRMS for the randomized portion of the clinical trial.
Patients will be randomized 1:1 using minimisation to account for age and sex by site.
Up to 400 PwRRMS for the observational cohort. The observational cohort will be compromised by participants not amenable to randomization, or who agree to randomization but are not approved for coverage for a medication in the arm to which they were randomized. The observational arm will be analyzed using propensity methods comparing participants started on EHT with those treated with an escalation treatment approach.
Study Population:
Adult men and women aged 18 to 60 years, inclusive, with established diagnosis of MS, as defined by the 2017 revision of McDonald Diagnostic Criteria, with RRMS. Participants must have evidence of active disease evidenced by one of: one or more MS relapses within the last 18 months prior to screening or radiological evidence of MS activity (≥2 new T2 lesions within the last 12 months from screening [compared to a previous recent MRI within 18 months of screening] or ≥1 GdE demonstrated on brain or spinal cord MRI performed within the last 12 months from screening). Enrolled participants must be ambulatory, EDSS ≤ 6.5, with disease onset ≤ 5 years and treatment-naïve (i.e., no MS DMT at any time in the past). Key exclusion criteria include: Prior use of MS DMT and other immunomodulators/immunosuppressive medications, participants with contraindications to disease modifying therapy; contraindications to MRI; or with clinically relevant medical or surgical conditions that, in the opinion of the investigator, would put the participant at risk by participating in the study.
Study Site:
24 Sites Total (TBD)
US Sites
- CCF (Cleveland) Coordinating
- CCF (Nevada)
- Ohio Health
- University of Colorado
- University of Rochester
- University of Texas, Houston
- University of Virginia
- University of Cincinnati
- Baylor College of Medicine
- University of Minnesota
- University of Wisconsin
UK Sites
- University of Nottingham Coordinating
- Cambridge University
- Morriston Hospital
- Oxford University
- Plymouth University
- Royal Gwent Hospital
- University of Cardiff
- University College London
- University of Glasgow
- Leeds General Infirmary
- Imperial College London
- University of Manchester
Treatment:
1) EHT arm: use of monoclonal antibodies (natalizumab, alemtuzumab, rituximab, or ocrelizumab) as first-line therapy
2) Escalation treatment arm: any other approved DMT as first-line with escalation of care based on clinical practice.
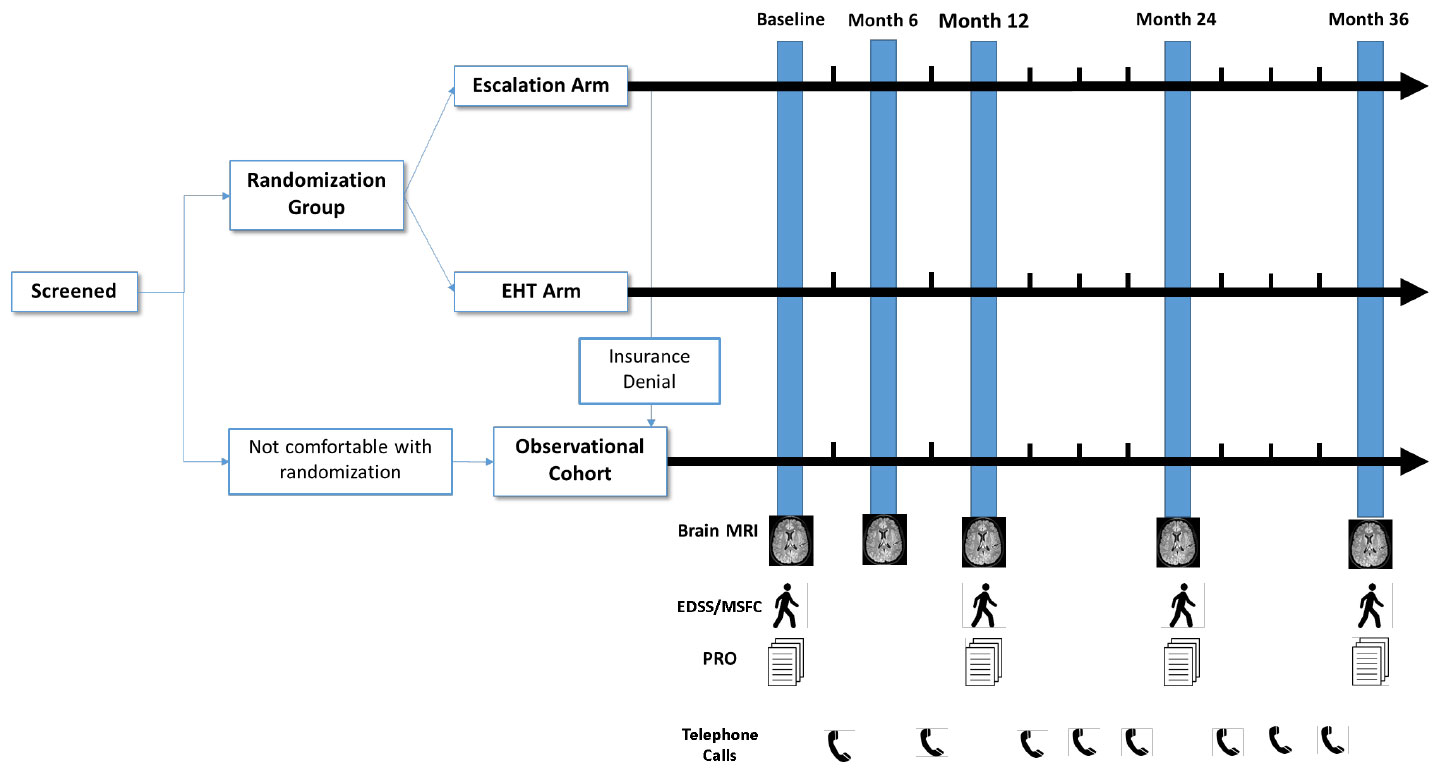
Visit Schedule:
Visits will include:
- Screening Visit and Randomization
- Randomization and Baseline Visit
- Month 6 visit
- Month 12 Visit
- Month 24 Visit
- Month 36 Visit
- Telephone visits at months 3, 9, 15, 18, 21, 27, 30, 33
Testing Schedule:
Testing will be performed according to the following: schedule:
- Safety assessments (AE review, concomitant therapy review, relapse review) at Baseline and Months 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36.
- Review of DMT at Months 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36.
- Review of relapses at Baseline and Months 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36.
- MRI of the brain at Baseline and Months 6, 12, 24, 36.
- Neurologic Assessments (EDSS, MSFC-4), and Patient-Reported Outcomes at Baseline and Months 12, 24, 36.
Safety Parameters:
The following safety measures will be employed in this study:
- Adverse event monitoring.
- Concomitant medication review.
Efficacy Parameters:
The following efficacy measures will be employed in this study:
MRI outcomes
- Normalized whole brain volume change
- T2-hyperintense lesion volume change/number
- T1-hypointense lesion volume change/number
- Gadolinium enhancing lesion volume/number
- Gray matter fraction change
Clinical outcomes
- Relapses
- EDSS
- MSFC-4
Patient reported outcomes
- MSIS-29
- Neuro-QoL
- PHQ-8
- TSQM
General Study Flow Chart
Study Schematic Diagram